14+ Chapter 12 Stoichiometry Answer Key
94 Effusion and Diffusion of Gases. 186 Occurrence Preparation and Properties of Carbonates.
![]()
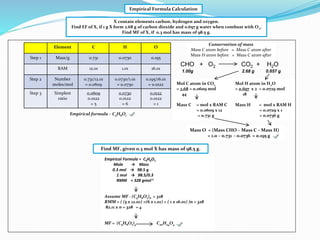
Class 11 Page 25 Balbharati Solutions
Web NCERT Solutions for Class 10 Maths Chapter 14.
. 96 Non-Ideal Gas Behavior. Web The electron configurations of silicon 14 electrons phosphorus 15 electrons sulfur 16 electrons chlorine 17 electrons and argon 18 electrons are analogous in the electron configurations of their outer shells to their corresponding family members carbon nitrogen oxygen fluorine and neon respectively except that the principal. The decomposition reaction exhibits first-order behavior at a quartz SiO 2 surface as suggested by the exponentially.
The term Stoichiometry was first coined or discovered by a German chemist named Jeremias Richter. Web NCERT Solutions for Class 10 Maths Chapter 14. UPSC Prelims 2022 Answer Key.
Web In 1824 at the age of 28 Nicolas Léonard Sadi Carnot Figure 167 published the results of an extensive study regarding the efficiency of steam heat enginesA later review of Carnots findings by Rudolf Clausius introduced a new thermodynamic property that relates the spontaneous heat flow accompanying a process to the temperature at which the process. 185 Occurrence Preparation and Compounds of Hydrogen. The larger the K a of an acid the larger the concentration of H 3 O H 3 O and A relative to the concentration of the nonionized acid HA in an equilibrium mixture and the stronger the.
French nobleman Antoine Lavoisier widely regarded as the father of modern chemistry changed chemistry from a qualitative to a quantitative science through his work with gasesHe discovered the law of conservation. 184 Structure and General Properties of the Nonmetals. NCERT Solutions For Class 9 Maths Chapter 13.
96 Non-Ideal Gas Behavior. NCERT Solutions For Class 9 Maths Chapter 13. 183 Structure and General Properties of the Metalloids.
Although water is a reactant in the reaction it is the solvent as well so we do not include H 2 O in the equation. An acid-base reaction is one in which a hydrogen ion H is transferred from one chemical species to anotherSuch reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial. NCERT Solutions for Class 10 Science.
Proposition 30 on reducing greenhouse gas emissions has lost ground in the past month with support among likely voters now falling short of a majority. Web where the concentrations are those at equilibrium. Web The molecular structure of the methane molecule CH 4 is shown with a tetrahedral arrangement of the hydrogen atomsVSEPR structures like this one are often drawn using the wedge and dash notation in which solid lines represent bonds in the plane of the page solid wedges represent bonds coming up out of the plane and dashed lines represent.
NCERT Solutions for Class 10 Science Chapter 1. Web A plot of A versus t for a zero-order reaction is a straight line with a slope of k and a y-intercept of A 0Figure 1211 shows a plot of NH 3 versus t for the thermal decomposition of ammonia at the surface of two different heated solids. BYJUS is Indias largest ed-tech company and the creator of Indias most loved school learning app.
Democrats hold an overall edge across the states competitive districts. 95 The Kinetic-Molecular Theory. Web Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more.
Mass of solute and volume of solution Asked for. Web 93 Stoichiometry of Gaseous Substances Mixtures and Reactions. To find the number of moles of CoCl 2 2H 2 O divide the.
It is 20 chapters in length and approximately 350-400 pages. 95 The Kinetic-Molecular Theory. The solution in Figure 1211 contains 100 g of cobaltII chloride dihydrate CoCl 2 2H 2 O in enough ethanol to make exactly 500 mL of solution.
Web It is expressed in unified atomic mass units represented by the unit symbol u. It is the most abundant substance on the surface of Earth and the only. The authors designed this textbook from the ground up to meet the needs of a one-semester course.
The balanced chemical equation provided equivalences that we used to construct conversion factorsFor example observe the following balanced chemical equation. 96 Non-Ideal Gas Behavior. The outcomes could determine which party controls the US House of Representatives.
The atomic mass is used to calculate the average mass of molecules and elements. UPSC Prelims 2022 Answer Key. Modification of work by vxlaFlickr.
However as noted previously in this chapter such conditions are not realistic. NCERT Solutions For Class 9 Maths Chapter 12. It is also used to solve the Stoichiometry problems.
In addition the technologies used to extract work from a spontaneous process eg batteries are never 100 efficient and so the work done by these processes is always less than the. The word stoichiometry is derived from the Greek word stoikhein meaning element and metron meaning measure. Web Key findings include.
NCERT Solutions for Class 10 Science. Web The Basics of General Organic and Biological Chemistry by David W. NCERT Solutions For Class 9 Maths Chapter 12.
What is the molar concentration of CoCl 2 2H 2 O. NCERT Solutions for Class 10 Science Chapter 1. 94 Effusion and Diffusion of Gases.
In general one atomic mass is equal to the 112 of the mass single carbon -12 atom. Hill and Rhonda J. Web When performing calculations stepwise as in Example 317 it is important to refrain from rounding any intermediate calculation results which can lead to rounding errors in the final resultIn Example 317 the molar amount of NaCl computed in the first step 1325 mol would be properly rounded to 132 mol if it were to be reported.
NCERT Solutions for Class 10 Science Chapter 1. Web Water H 2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid which is nearly colorless apart from an inherent hint of blueIt is by far the most studied chemical compound and is described as the universal solvent and the solvent of life. Web Chemical Stoichiometry refers to the quantitative study of the reactants and products involved in a chemical reaction.
Modification of work by the Italian voiceFlickr. Web The study of the chemical behavior of gases was part of the basis of perhaps the most fundamental chemical revolution in history. 95 The Kinetic-Molecular Theory.
Web 93 Stoichiometry of Gaseous Substances Mixtures and Reactions. Web 93 Stoichiometry of Gaseous Substances Mixtures and Reactions. Web In Chapter 5 Stoichiometry and the Mole we related quantities of one substance to another in a chemical equation by performing calculations that used the balanced chemical equation.
94 Effusion and Diffusion of Gases. Web where w max w max refers to all types of work except expansion pressure-volume work. UPSC Prelims 2022 Answer Key.
182 Occurrence and Preparation of the Representative Metals. Scott is for the one-semester General Organic and Biological Chemistry course. Web NCERT Solutions for Class 10 Maths Chapter 14.
Launched in 2015 BYJUS offers highly personalised and effective learning programs for classes 1 - 12 K-12 and aspirants of competitive exams like. NCERT Solutions for Class 10 Science. NCERT Solutions For Class 9 Maths Chapter 13.
NCERT Solutions For Class 9 Maths Chapter 12.

Pdf Magnetic Behavior And Raman Spectroscopy Of The Composite System Of Cucl 2 2h 2 O C 12 H 9 No
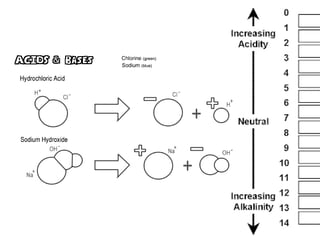
2 6 Acids Bases And Salts

Chgrt12

Chem Int Cc Ch 12 Stoichiometry Answers 09 15 Pdf Ck 12 Chemistry Concepts Intermediate Answer Key Chapter 12 Stoichiometry 12 1 Everyday Course Hero

Mole Conversions One Step Worksheet

Lots Of Definitions To Learn Isotopes Isotopes The Mole The Mole Avogadro Number Avogadro Number Relative Atomic Molecular Mass Relative Atomic Molecular Ppt Download

Stoichiometry Exercises Worksheet
Class 11 Page 25 Balbharati Solutions
Chapter 11 Stoichiometry 11 1 Defining Stoichiometry

Mole Conversions One Step Worksheet

Chgrt12
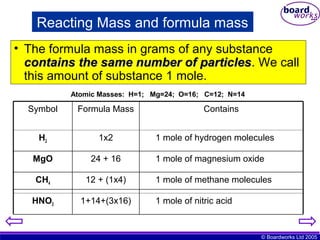
Ks4 Quantitative Chemistry Boardworks

Chapter 12 Stoichiometry Flashcards Quizlet

Chapter 12 Stoichiometry Flashcards Quizlet

Chgrt12

Chapter 12 Stoichiometry Ppt Video Online Download

Day 06 Stoichiometry Worksheet 1 And 2 Answers Pdf Stoichiometry Worksheet I 0 I 0 0 L Name 0 Section Due Date 1 Ammonia Gas Course Hero